MEDSynth's significant publications

2024
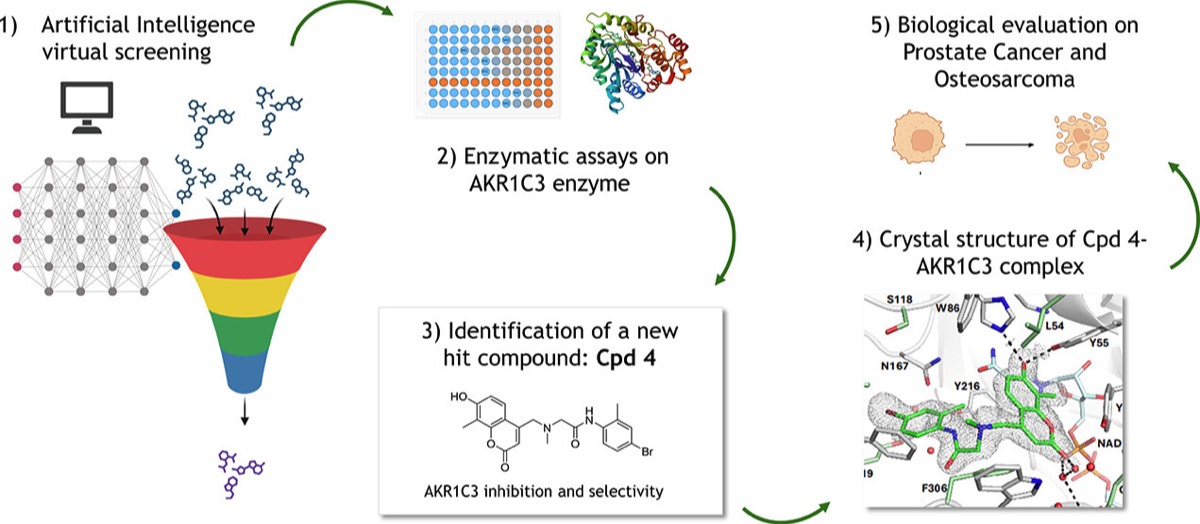
A. C. Pippione, C. Vigato, C. Tucciarello, S. Hussain, E. Salladini, H. H. Truong, Niel M Henriksen, G. Vanzetti, G. Giordano, D. Zonari, O. A. Mirza, K. Frydenvang, Y. Pignochino, S. Oliaro-Bosso, D. Boschi and M. L. Lolli. AI-based discovery of a new AKR1C3 inhibitor for anticancer applications. ACS Medicinal Chemistry Letters. https://doi.org/10.1021/acsmedchemlett.4c00150
- M. Alberti, G. Poli, L. Broggini, S. Sainas, M. Rizzi, D. Boschi, D. M. Ferraris, E. Martino, S. Ricagno, T. Tuccinardi, M. L. Lolli and R. Miggiano. An alternative conformation of the N-terminal loop of human dihydroorotate dehydrogenase drives binding to a potent antiproliferative agent. Acta Cryst. 2024, D80, 386-396. https://doi.org/10.1107/S205979.
- J. S. Grossert, A. M. J. Crowell, D. Boschi, M. L. Lolli, R. L. White. Tandem mass spectrometry of homologous 3-hydroxyfurazan and nitrile amino acids: Analysis of cooperative interactions and fragmentation processes. J. Mass Spectrom. 2024, 59, 5043. https://doi.org/10.1002/jms.5043.
- M. Sharma, V. Pandey, G. Poli, T. Tuccinardi, M. L. Lolli, V. K. Vyas. A comprehensive review of synthetic strategies and SAR studies for the discovery of PfDHODH inhibitors as antimalarial agents. Part 1: triazolopyrimidine, isoxazolopyrimidine and pyrrole-based (DSM) compounds. Bioorg Chem. 2024, 146, 107249. doi: 10.1016/j.bioorg.2024.107249.
- A. C. Pippione, S. Kovachka, C. Vigato, L. Bertarini, I. Mannella, S. Sainas, B. Rolando, E. Denasio, H. Piercy-Mycock , L. Romalho, E. Salladini, S. Adinolfi, D. Zonari, C. Peraldo-Neia, G. Chiorino, A. Passoni, O. A. Mirza , K. Frydenvang, K. Pors, M. L. Lolli, F. Spyrakis, S. Oliaro-Bosso,D. Boschi. Structure-guided optimization of 3-hydroxybenzoisoxazole derivatives as inhibitors of Aldo-keto reductase 1C3 (AKR1C3) to target prostate cancer. Eur. J. Med. Chem. 2024, 268, 116193. https://doi.org/10.1016/j.ejmech.2024.116193.

- J. S. Grossert, D. Boschi, M. L. Lolli, R. L. White. Intramolecular interactions and the neutral loss of ammonia from collisionally activated, protonated ω-aminoalkyl-3-hydroxyfurazans. Eur J Mass Spectrom (Chichester). 2024, 30(1), 38–46. doi: 10.1177/14690667231214672
2023
- A. Luganini, G. Sibille, M. Pavan, M. Mello Grand; S. Sainas, D. Boschi , M. L. Lolli , G. Chiorino, G. Gribaudo. Mechanisms of antiviral activity of the new hDHODH inhibitor MEDS433 against respiratory syncytial virus replication. Antivir, Res. 2023, 219, 105734. https://doi.org/10.1016/j.antiviral.2023.105734
- T. M Wrobel, K. Sharma, I. Mannella, S. Oliaro-Bosso, P. Nieckarz, T. Du Toit, C. D. Voegel, M. N. Rojas Velazquez, J. Yakubu, A. Matveeva, S. Therkelsen, F. Steen Jorgensen, A. V. Pandey, A. C. Pippione, M. L. Lolli, D. Boschi, F. Bjorkling. Exploring the Potential of Sulfur Moieties in Compounds Inhibiting Steroidogenesis. Biomolecules. 2023, 13(9), 1349. https://doi.org/10.3390/biom13091349
- M. Alberti, S. Sainas, E. Ronchi, M. L. Lolli, D. Boschi, M. Rizzi, D, M. Ferraris, R. Miggiano. Biochemical characterization of Mycobacterium tuberculosis dihydroorotate dehydrogenase and identification of a selective inhibitor. 2023, 597, 2119-2132. https://doi.org/10.1002/1873-3468.14680
- A. Kumar, C. Vigato, D. Boschi, M.L. Lolli, D. Kumar. Phenothiazines as anti-cancer agents: SAR overview and synthetic strategies. Eur. J.Med. Chem. 2023, 254, 115337. https://doi.org/10.1016/j.ejmech.2023.115337
2022
- A.C. Pippione, Z. Kilic-Kurt, S. Kovachka, S. Sainas, B. Rolando, E. Denasio, K. Pors, S. Adinolfi, D. Zonari, R. Bagnati, M.L. Lolli, F. Spyrakis, S. Oliaro-Bosso, D. Boschi. New aldo-keto reductase 1C3 (AKR1C3) inhibitors based on the hydroxytriazole scaffold. Eur. J. Med. Chem. 2022, 237, 114366. DOI:10.1016/j.ejmech.2022.114366
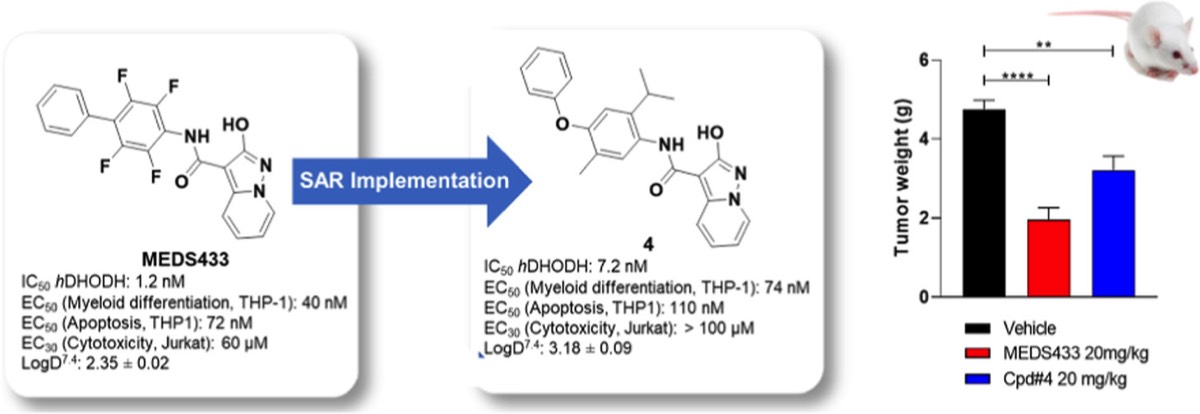
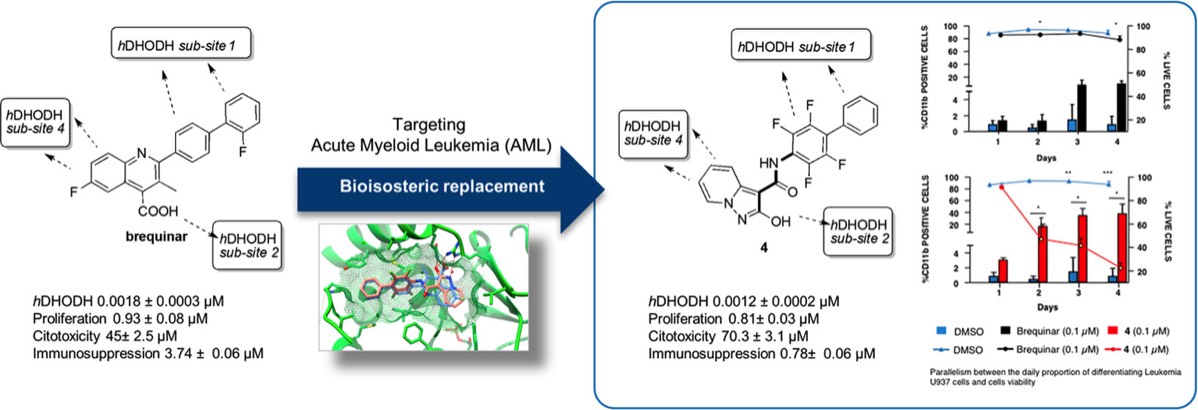
- S. Sainas, M. Giorgis, P. Circosta, G. Poli, M. Alberti, A. Passoni, V. Gaidano, A.C. Pippione, N. Vitale, D. Bonanni, B. Rolando, A. Cignetti, C. Ramondetti, A. Lanno, D.M. Ferraris, B. Canepa, B. Buccinnà, M. Piccinini, M. Rizzi, G. Saglio, S. Al-Karadaghi, D. Boschi, R. Miggiano, T. Tuccinardi, M.L. Lolli. Targeting Acute Myelogenous Leukemia Using Potent Human Dihydroorotate Dehydrogenase Inhibitors Based on the 2-Hydroxypyrazolo[1,5-a ]pyridine Scaffold: SAR of the Aryloxyaryl Moiety J. Med. Chem. 2022, 65 (19), 12701-12724. https://pubs.acs.org/doi/10.1021/acs.jmedchem.2c00496
2021
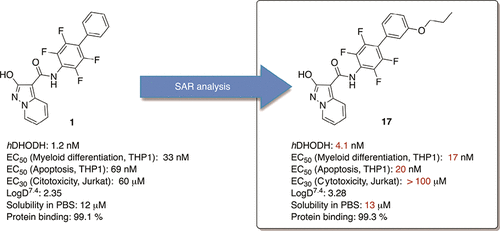
- Sainas, S.; Giorgis, M.; Circosta, P.; Gaidano, V.; Bonanni, D.; Pippione, A. C.; Bagnati, R.; Passoni, A.; Qiu, Y.; Cojocaru, C. F.; Canepa, B.; Bona, A.; Rolando, B.; Mishina, M.; Ramondetti, C.; Buccinnà, B.; Piccinini, M.; Houshmand, M.; Cignetti, A.; Giraudo, E.; Al-Karadaghi, S.; Boschi, D.; Saglio, G.; Lolli, M. L. Targeting Acute Myelogenous Leukemia Using Potent Human Dihydroorotate Dehydrogenase Inhibitors Based on the 2-Hydroxypyrazolo[1,5-a]Pyridine Scaffold: SAR of the Biphenyl Moiety. J. Med. Chem. 2021, 64 (9), 5404–5428.https://doi.org/10.1021/acs.jmedchem.0c01549
- Luganini, A.; Sibille, G.; Mognetti, B.; Sainas, S.; Chiara, A.; Giorgis, M.; Boschi, D.; Lolli, M. L.; Gribaudo, G. Effective Deploying of a Novel DHODH Inhibitor against Herpes Simplex Type 1 and Type 2 Replication. Antiviral Res. 2021, 189 (October 2020), 105057. https://doi.org/10.1016/j.antiviral.2021.105057
- Pippione, A.C., Sainas, S., Boschi, D., Lolli, M.L. Hydroxyazoles as acid isosteres and their drug design applications—Part 2: Bicyclic systems. Advances in Heterocyclic Chemistry, 2021 https://doi.org/10.1016/bs.aihch.2020.12.002
- Gaidano,V.; Houshmand, M.; Vitale, N.; Carrà, G.; Morotti, A.; Tenace, V.; Rapelli, S.; Sainas, S.; Pippione, A.C.; Giorgis, M.; et al. The Synergism between DHODH Inhibitors and Dipyridamole Leads to Metabolic Lethality in Acute Myeloid Leukemia. Cancers. 2021, 13, 1003. https://doi.org/10.3390/cancers13051003
2020
- Ferrante, T., Adinolfi, S., D'Arrigo, G., Poirier, D., Daga, M., Lolli, M.L., Balliano, G., Spyrakis, F., Oliaro-Bosso, S. Multiple catalytic activities of human 17β-hydroxysteroid dehydrogenase type 7 respond differently to inhibitors. Biochimie. 2020, 170, 106-117 https://doi.org/10.1016/j.biochi.2019.12.012
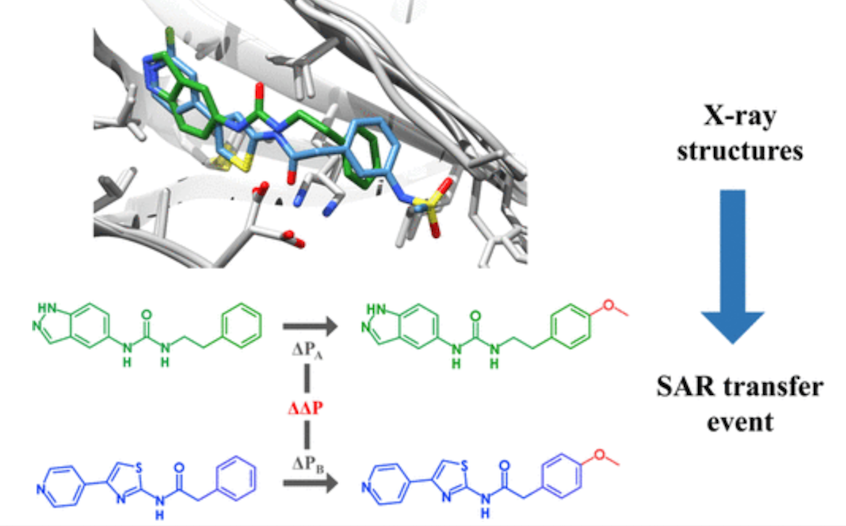
- Bonanni, D., Lolli, M.L., Bajorath, J. Computational Method for Structure-Based Analysis of SAR Transfer. Journal of Medicinal Chemistry. 2020, 63(3), 1388-1396 https://doi.org/10.1021/acs.jmedchem.9b01931
2019
- Boschi, D., Pippione, A.C., Sainas, S., Lolli, M.L. Dihydroorotate dehydrogenase inhibitors in anti-infective drug research. European Journal of Medicinal Chemistry, 2019, 183, 111681 https://doi.org/10.1016/j.ejmech.2019.111681
- Sainas, S., Pippione, A.C., Giraudo, A., Martina, K., Bosca, F., Rolando, B., Barge, A., Ducime, A., Federico, A., Grossert, S.J., White, R.L., Boschi, D., Lolli, M.L. Regioselective N-Alkylation of Ethyl 4-Benzyloxy-1,2,3-triazolecarboxylate: A Useful Tool for the Synthesis of Carboxylic Acid Bioisosteres. Journal of Heterocyclic Chemistry, 2019, 56(2), 501-519 https://doi.org/10.1002/jhet.3426
- Pippione, A.C., Sainas, S., Goyal, P., Fritzson, I., Cassiano, G.C., Giraudo, A., Giorgis, M., Tavella, T.A., Bagnati, R., Rolando, B., Caing-Carlsson, R., Costa, F.T.M., Andrade, C.H., Al-Karadaghi, S., Boschi, D., Friemann, R., Lolli, M.L. Hydroxyazole scaffold-based Plasmodium falciparum dihydroorotate dehydrogenase inhibitors: Synthesis, biological evaluation and X-ray structural studies. European Journal of Medicinal Chemistry, 2019, 163, 266-280 https://doi.org/10.1016/j.ejmech.2018.11.044
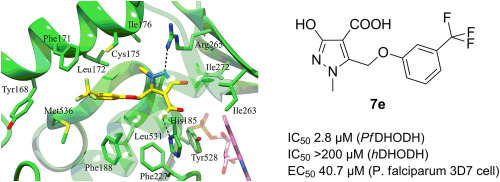
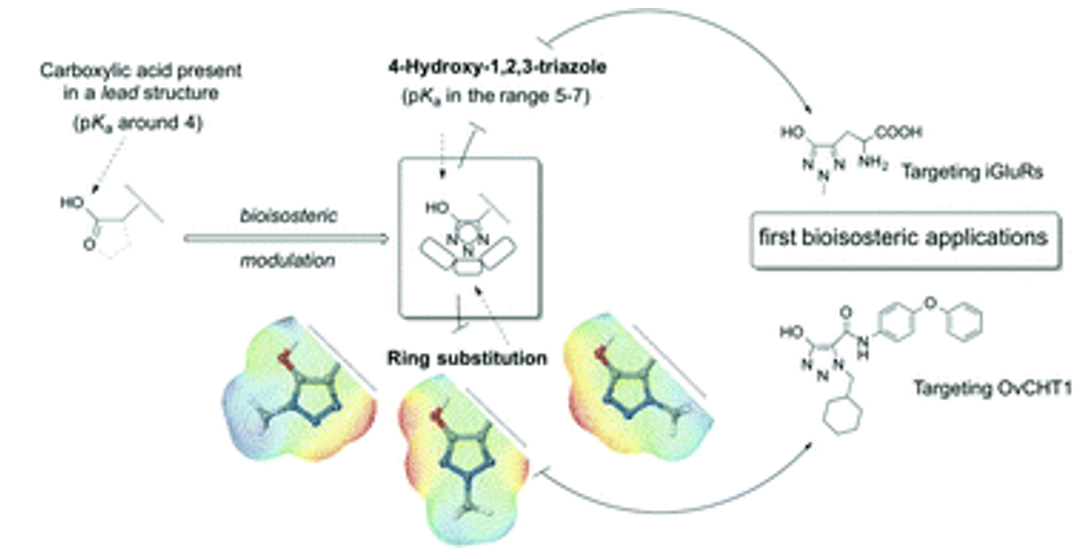
- Sainas, S., Temperini, P., Farnsworth, J.C., Yi, F., Møllerud, S., Jensen, A.A., Nielsen, B., Passoni, A., Kastrup, J.S., Hansen, K.B., Boschi, D., Pickering, D.S., Clausen, R.P., Lolli, M.L. Use of the 4-Hydroxytriazole Moiety as a Bioisosteric Tool in the Development of Ionotropic Glutamate Receptor Ligands. Journal of Medicinal Chemistry, 2019, 62(9), 4467-4482 https://doi.org/10.1021/acs.jmedchem.8b01986
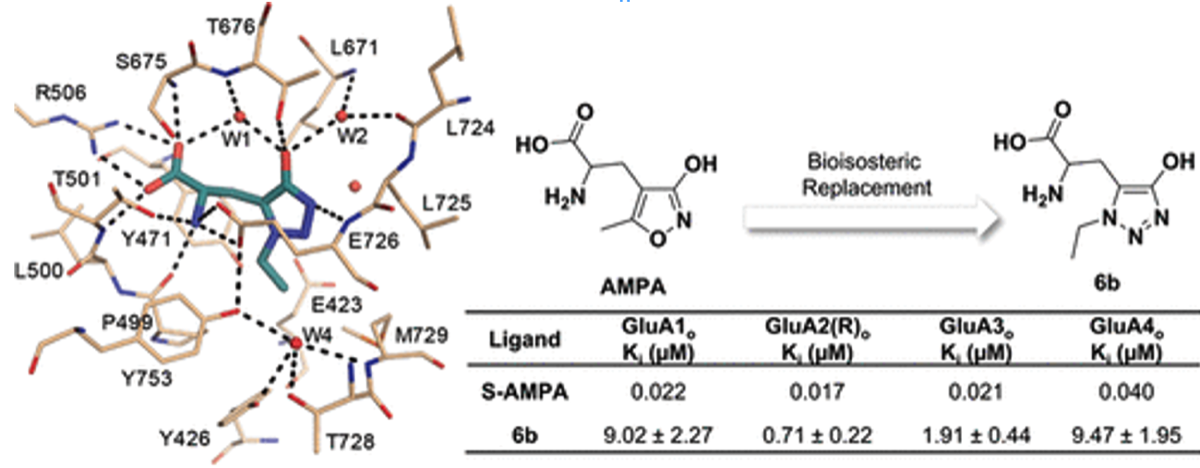
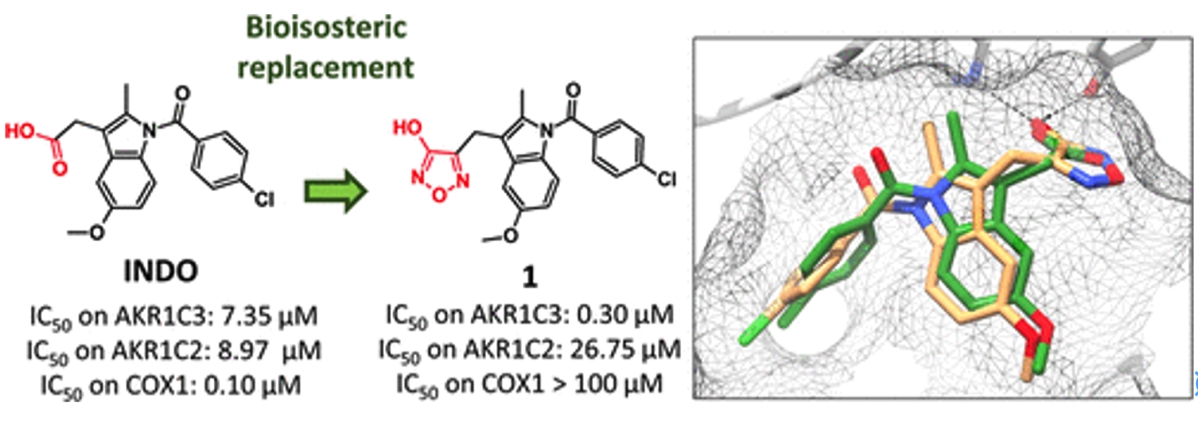
- Lolli, M.L., Carnovale, I.M., Pippione, A.C., Wahlgren, W.Y., Bonanni, D., Marini, E., Zonari, D., Gallicchio, M., Boscaro, V., Goyal, P., Friemann, R., Rolando, B., Bagnati, R., Adinolfi, S., Oliaro-Bosso, S., Boschi, D.Bioisosteres of Indomethacin as Inhibitors of Aldo-Keto Reductase 1C3 . ACS MED. CHEM. LETT. 2019, 10 (4), 437-443 https://doi.org/10.1021/acsmedchemlett.8b00484
2018
Giraudo, A. Krall, J., Nielsen, B., Sørensen, T.E., Kongstad, K.T., Rolando, B., Boschi, D, Frølund, B.* and Lolli, M.L. * 4-Hydroxy-1,2,3-triazole moiety as bioisostere of the carboxylic acid function: a novel scaffold to probe the orthosteric γ-aminobutyric acid receptor binding site. Eu. J. Med. Chem., 2018, 158, 311-321 https://doi.org/10.1016/j.ejmech.2018.08.094
Grossert, J.S., Boschi, D., Lolli, M.L., White, R.L. Fragmentation pathways arising from protonation at different sites in aminoalkyl-substituted 3-hydroxy-1,2,5-oxadiazoles (3-hydroxyfurazans). Rapid Communications in Mass Spectrometry, 2018, 32(16),1403-1413. https://doi.org/10.1002/rcm.8166
Sainas, S., Pippione, A.C., Boschi, D., Gaidano, V., Circosta, P., Cignetti, A., Dosio, F., Lolli, M.L. DHODH inhibitors and leukemia: An emergent interest for new myeloid differentiation agents. Drugs of the Future, 2018, 43 (11), 823-834 DOI: 10.1358/dof.2018.043.11.2856492
Marco L. Lolli, Stefano Sainas, Agnese C. Pippione, Marta Giorgis, Donatella Boschi and Franco Dosio* Use of human Dihydroorotate Dehydrogenase (hDHODH) Inhibitors in Autoimmune Diseases and New Perspectives in Cancer Therapy. Recent Patents on Anti-Cancer Drug Discovery 2018, 13(1), 218. DOI:10.2174/1574892812666171108124218
Sainas, S., Dosio, F., Boschi, D., Lolli, M.L.* Targeting Human Onchocerciasis: Recent Advances Beyond Ivermectin. Annual Reports in Medicinal Chemistry, 2018, 51, 1-38 https://doi.org/10.1016/bs.armc.2018.08.001
Sanchez, Elena; Artuso, Emma; Lombardi, Chiara; Visentin, Ivan; Lace, Beatrice; Saeed, Wajeeha; Lolli, Marco L.; Kobauri, Piermichele; Ali, Zahid; Spyrakis, Francesca; Cubas, Pilar; Cardinale, Francesca; Prandi, Cristina New insights into Structure Activity Relationship of strigolactones via a novel, quantitative in planta bioassay. J. Exp. Botany, 2018, 69(9), pp. 2333-2343 https://doi.org/10.1093/jxb/ery092
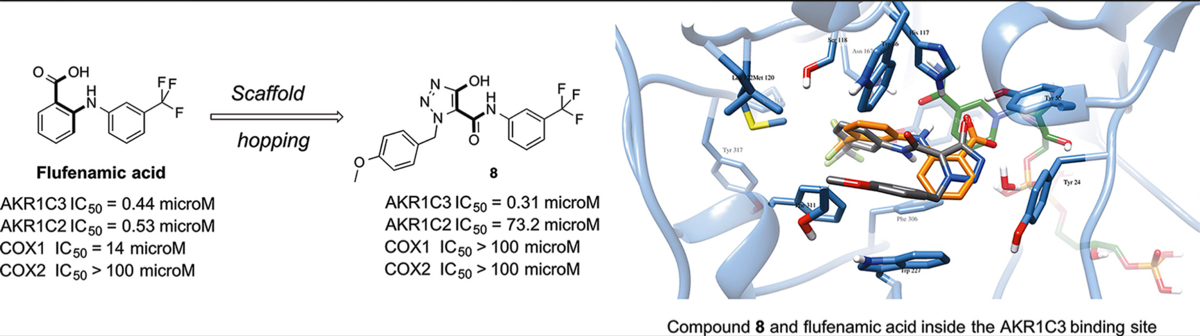
Pippione, Agnese Chiara, Carnovale, Irene Maria, Bonanni, Davide, Sini, Marcella, Goyal, Parveen, Marini, Elisabetta, Pors, Klaus, Adinolfi, Salvatore, Zonari, Daniele, Festuccia, Claudio, Wahlgren, Weixiao Yuan, Friemann, Rosmarie, Bagnati, Renzo, Boschi, Donatella, Oliaro-Bosso, Simonetta, * and Lolli, Marco Lucio* Potent and selective aldo-keto reductase 1C3 (AKR1C3) inhibitors based on the benzoisoxazole moiety: Application of a bioisosteric scaffold hopping approach to flufenamic acid. Eu. J. Medicinal Chemistry, 2018, 150, 930-945 https://doi.org/10.1016/j.ejmech.2018.03.040
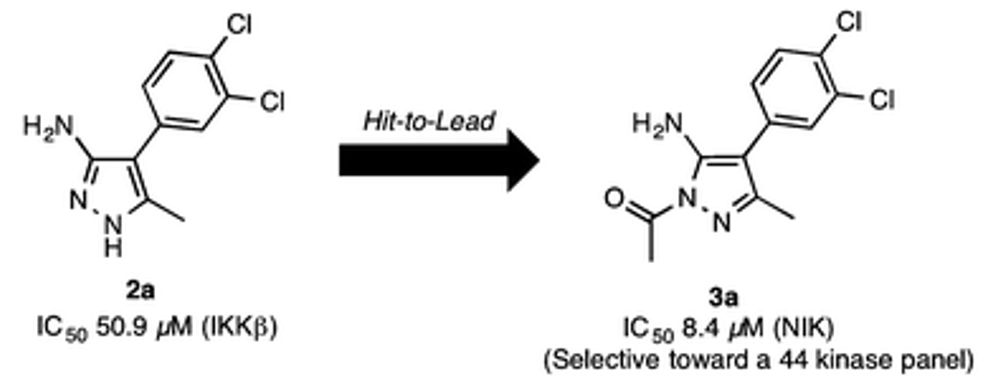
- Agnese C. Pippione, Stefano Sainas, Antonella Federico, Elisa Lupino, Marco Piccinini, Michael Kubbutat, Jean-Marie Contreras, Christophe Morice, Alessandro Barge, Alex Ducime, Donatella Boschi, Salam Al-Karadaghi and Marco L. Lolli. N-Acetyl-3-aminopyrazoles block the non canonical NF-kB cascade by selectively inhibiting NIK. MedChemComm, 2018, 9, 963 - 968 https://doi.org/10.1039/C8MD00068A
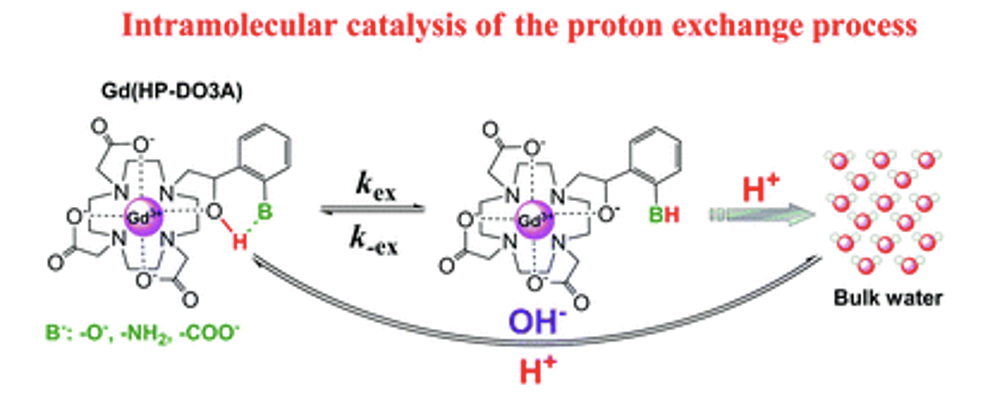
Irene Maria Carnovale, Marco Lucio Lolli, Sonia Colombo Serra, Alberto Fringuello Mingo, Roberta Napolitano, Valeria Boi, Nicol Guidolin, Luciano Lattuada, Fabio Tedoldi, Zsolt Baranyai* and Silvio Aime* Exploring the intramolecular catalysis of the proton exchange process to modulate the relaxivity of Gd(III)-complexes of HP-DO3A-like ligands. Chem Comm, 2018, 54, 72, 2018, 10056-10059 https://doi.org/10.1039/C8CC05284K
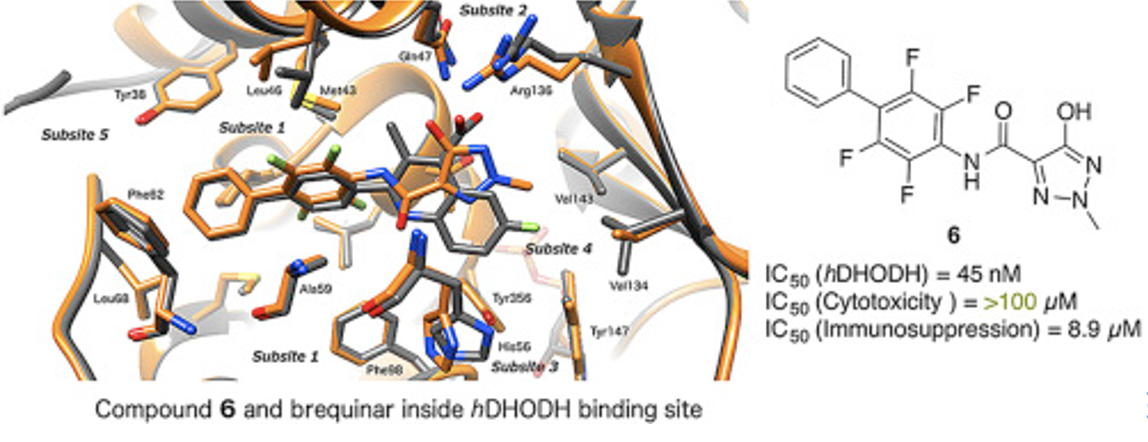
- Sainas, Stefano; Pippione, Agnese; Lupino, Elisa; Giorgis, Marta; Circosta, Paola; Gaidano, Valentina; Goyal, Parveen; Bonanni, Davide; Rolando, Barbara; Cignetti, Alessandro; Ducime, Alex; Andersson, Mikael; Järvå, Michael; Friemann, Rosmarie; Piccinini, Marco; Ramondetti, Cristina; Buccinnà, Barbara; Al-Karadaghi, Salam; Boschi, Donatella; Saglio, Giuseppe; Lolli, Marco* Targeting myeloid differentiation using potent 2-hydroxypyrazolo[1,5-a]pyridine scaffold-based human dihydroorotate dehydrogenase (hDHODH) inhibitors. J. Medicinal Chemistry, 2018, 61 (14), 6034-6055. https://doi.org/10.1021/acs.jmedchem.8b00373
2017
- Pippione, A. C.; Boschi, D.; Pors, K.; Oliaro-Bosso, S.; Lolli, M. L. Androgen-AR axis in primary and metastatic prostate cancer: chasing steroidogenic enzymes for therapeutic intervention. Journal of Cancer Metastasis and Treatment, 2017, 3, 328-61 DOI:10.20517/2394-4722.2017.44
- Agnese C. Pippione, Antonella Federico, Alex Ducime, Stefano Sainas, Donatella Boschi, Alessandro Barge, Elisa Lupino, Marco Piccinini, Michael Kubbutat, Jean-Marie Contreras, Christophe Morice, Salam Al-Karadaghi and Marco L. Lolli.* 4-Hydroxy-N-[3,5-bis(trifluoromethyl)phenyl]- 1,2,5-thiadiazole-3-carboxamide: a novel inhibitor of the canonical NF-kB cascade. Med. Chem. Commun., 2017, 8, 1850-1855 https://doi.org/10.1039/C7MD00278E
- Elena Campaner*, Alessandra Rustighi*, Alessandro Zannini, Alberto Cristiani, Silvano Piazza, Yari Ciani, Ori Kalid, Gali Golan, Erkan Baloglu, Sharon Shacham, Barbara Valsasina, Ulisse Cucchi, Agnese Chiara Pippione, Marco Lucio Lolli, Barbara Giabbai, Paola Storici, Paolo Carloni, Giulia Rossetti, Federica Benvenuti, Ezia Bello, Maurizio D’Incalci, Elisa Cappuzzello, Antonio Rosato & Giannino Del Sal. A covalent PIN1 inhibitor selectively targets cancer cells by a dual mechanism of action . Nature Communications, 2017, (8), 15772. https://doi.org/10.1038/ncomms15772
- Agnese C. Pippione, Alessandro Giraudo, Davide Bonanni, Irene M. Carnovale, Elisabetta Marini, Clara Cena, Annalisa Costale, Daniele Zonari, Klaus Pors, Maria Sadiq, Donatella Boschi, Simonetta Oliaro-Bosso* and Marco L. Lolli.* Hydroxytriazole derivatives as potent and selective aldo-keto reductase 1C3 (AKR1C3) inhibitors discovered by bioisosteric scaffold hopping approach. Eu. J. Med. Chem. 2017, 139, 936-946. https://doi.org/10.1016/j.ejmech.2017.08.046
- Sainas Stefano, Pippione Agnese C., Giorgis Marta, Lupino Elisa, Goyal Parveen, Ramondetti Cristina, Buccinnˆ Barbara, Piccinini Marco, Braga Rodolpho C., Andrade Carolina H., Andersson Mikael, Moritzer Ann-Christin, Friemann Rosmarie, Mensa Stefano, Al-Karadaghi Salam, Boschi Donatella, Lolli Marco L. Design, synthesis, biological evaluation and X-ray structural studies of potent human dihydroorotate dehydrogenase inhibitors based on hydroxylated azole scaffolds. Eur. J. Med. Chem. 2017, 129, 287-302, doi: 10.1016/j.ejmech.2017.02.017
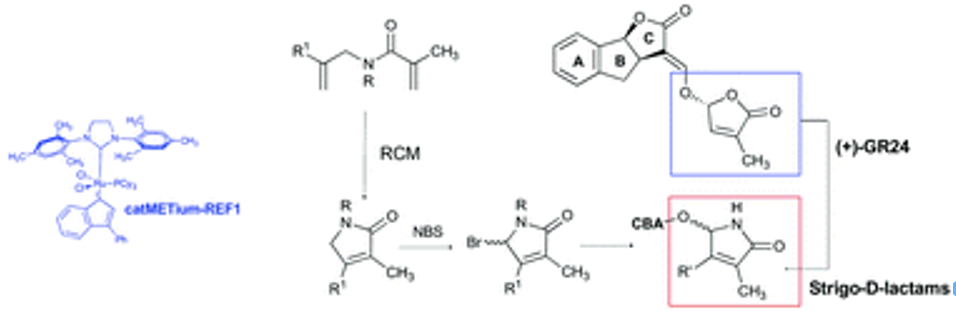
- Chiara Lombardi, Emma Artuso, Eleonora Grandi, Marco Lolli, Francesca Spirakys, Emanuele Priola, and Cristina Prandi Synthesis of Analogues of Phytohormones Strigolactones with Ring-Closing Metathesis as a Key Step. Org. Biomol. Chem. 2017, 15, 8218-8231. https://doi.org/10.1039/C7OB01917C
2015
- J. Stuart Grossert, Agnese C. Pippione, Donatella Boschi, Marco L. Lolli, Robert L. White. Heterocyclic ring cleavage upon collision-induced dissociation of deprotonated 3-hydroxy-1,2,5-oxadiazoles (3-hydroxyfurazans). Journal of Mass Spectrometry. 2015, 50, p. 1433-1437, ISSN: 1096-9888, https://doi.org/10.1002/jms.3724
- Marco Lolli, Sarah Narramore, Colin W.G. Fishwick and Klaus Pors. Refining the chemical toolbox for drug discovery in the 21st Century. Drug Discovery of Today 2015, 20(8), 1018 - 1026. https://doi.org/10.1016/j.drudis.2015.04.010
- Cristian Taccioli, Giovanni Sorrentino, Alessandro Zannini, Jimmy Caroli, Domenico Beneventano, Laura Anderlucci, Naomi Ruggeri, Marco Lolli, Silvio Bicciato and Giannino Del Sal. MDP, a database linking drug response data to genomic information, identifies Dasatinib and Statins as a combinatorial strategy to inhibit YAP/TAZ in cancer cells. Oncotarget 2015, 10, 6(36), 38854 doi: 10.1007/s00894-012-1643-5
- Agnese C. Pippione, Franco Dosio, Alex Ducime, Antonella Federico, Katia Martina, Stefano Sainas, Bente Frolund, Major Gooyit, Kim D. Janda, Donatella Boschi and Marco L. Lolli. Substituted 4-hydroxy-1,2,3-triazoles: synthesis, characterization and first drug design applications through bioisosteric modulation and scaffold hopping approaches. Med. Chem. Commun., 2015, 6, 1285-1292. https://doi.org/10.1039/C5MD00182J
Other representative publications
- Silvia Bonomo, Paolo Tosco, Marta Giorgis, Marco Lolli, Roberta Fruttero The role of fluorine in stabilizing the bioactive conformation of dihydroorotate dehydrogenase inhibitors. Journal of Molecular Modeling 2013, 19(3), 1099-1107 doi: 10.1007/s00894-012-1643-5
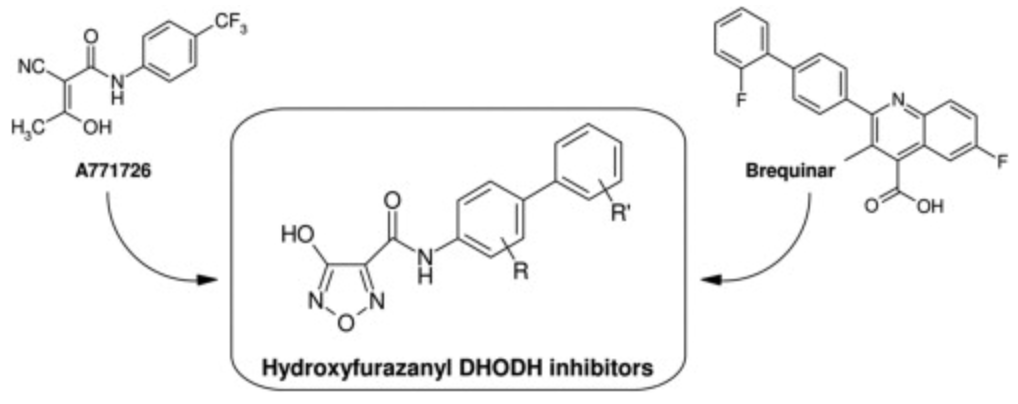
- Marco L. Lolli, Marta M. Giorgis, Paolo P. Tosco, Antonio A Foti, Roberta R. Fruttero and Alberto A. Gasco New inhibitors of dihydroorotate dehydrogenase (DHODH) based on the 4-hydroxy-1,2,5-oxadiazol-3-yl (hydroxyfurazanyl) scaffold. Eur J Med Chem 2012, 49, 102-9 doi: 10.1016/j.ejmech.2011.12.038
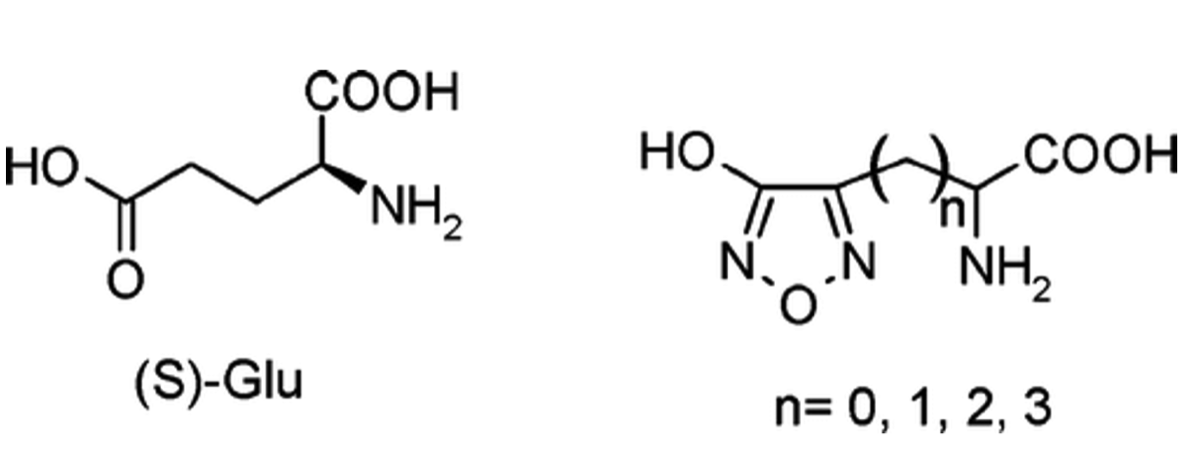
- Lolli, M. L.; Hansen, S. L.; Rolando, B.; Nielsen, B.; Wellendorph, P.; Madsen, K.; Larsen, O. M.; Kristiansen, U.; Fruttero, R.; Gasco, A.; Johansen, T. N. Hydroxy-1,2,5-oxadiazolyl Moiety as Bioisoster of the Carboxy Function. Synthesis, Ionization Constants, and Pharmacological Characterization of gamma-Aminobutyric Acid (GABA) Related Compounds. J. Med. Chem., 2006, 49(14), 4442 - 4446 https://doi.org/10.1021/jm051288b